In February 2019, a news story went viral on social media highlighting the detrimental effects of an acidic diet. A young man, with a habit of drinking six Monster Energy drinks every day, for 7 months, ended up with significant irreversible erosive damage to his teeth.
https://nypost.com/2019/02/13/energy-drinks-completely-ruined-my-life/
An outlier that made for an eye-catching headline for sure, but it does highlight the potential damage acidity can cause to our teeth if left unmanaged.
Foods, oral hygiene products, even our own saliva, all contribute to the chemistry of our oral environment. We have all had the experience of drinking an acidic beverage (e.g. orange juice) or a carbonated beverage (e.g. bubbly water) and having our teeth surface feel rough to our tongue after. Microscopically, what happens is the acidity had removed the surface layer of the tooth structure – exposing the microscopic surface pores underneath. It is the same process dentists use when we do a filling restoration – we use phosphoric acid gel to “etch” and expose the surface pores for better bonding of the filling material.
Pronamel has an excellent resource page on the scale of acidity in everyday foods.
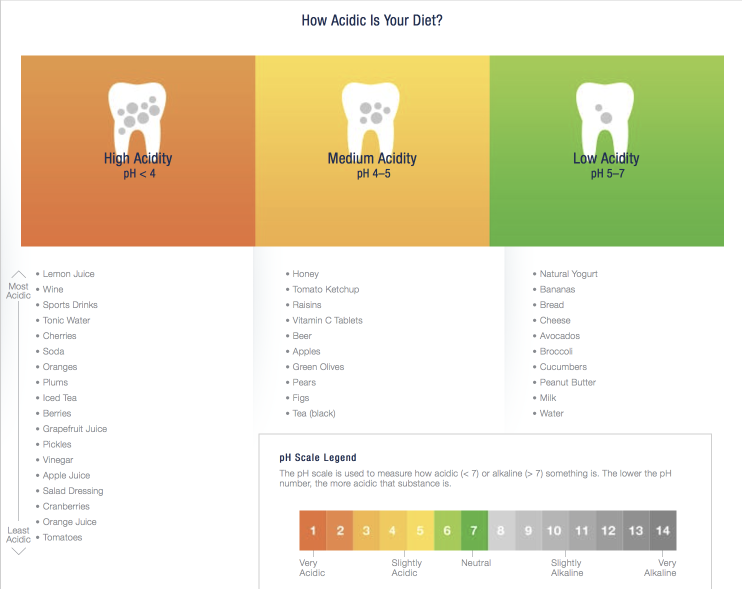
https://www.pronamel.us/tooth-erosion/causes-of-acid-erosion/
It is also important to note that not all oral hygiene products are created equal. Some mouth rinses, while having formulations able to improve gum health, also have the same acidity levels as orange juice. Studies have shown that prolonged use of acidic mouth rinses can cause erosive damage to enamel1 so it is important to choose one with neutral or alkaline pH.
http://kincardinedentistry.com/wp-content/uploads/2014/12/Rinse-pH-Values.pdf
Revisiting the example of the damage by the Monster Energy drinks, oral hygiene was most definitely a contributory factor. In addition to erosion, cavities also result from chemistry imbalance. In the oral environment, there is a constant balance of demineralization (Breakdown) and remineralization (Re-building) on the tooth surface. Cavities are formed when the scale is tipped toward breakdown.
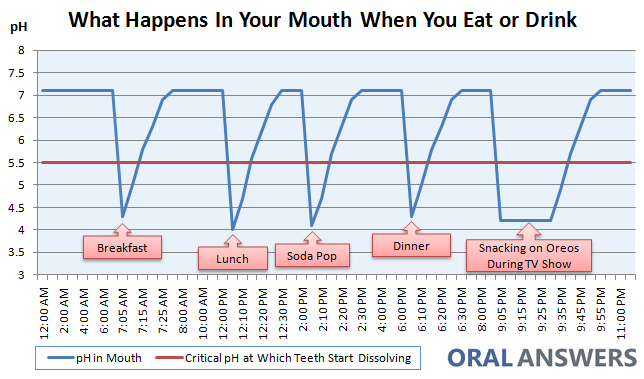
The Stephan curve is a useful resource to understand the demineralization/remineralization process. Every time food enters your mouth, you experience an increase in your salivary levels.
In addition to the mechanical chewing/breakdown of foods with your teeth, enzymes in your saliva breaks down the carbohydrate structure on a microscopic level to aid in digestion. Overall, the presence of carbohydrates increases the acidity (lowers the pH). After a meal, other components of saliva (calcium, phosphate, fluoride, and other electrolytes) buffer and reverse the acidic environment back to neutral.
Certain risk factors can tip the balance, such as:
- Inadequate oral hygiene leading to plaque accumulation – imagine clumps of acid-producing bacteria sitting on the surface of the tooth, left undisturbed by a toothbrush or floss
- Bacterial biofilm imbalance – having a higher population of the acid-producing bacteria that causes cavities
- Amount of saliva – less saliva means less potential for remineralization and more demineralization
- Medication or medical conditions that leads to dry mouth
- Acidity from gastric reflux
- Diet
Compare a person who eats 3 meals a day to a person who eats the same amount of food over 4-5 sittings and/or also snacks/”grazes” at his/her office desk. The longer the duration food is present in the mouth, the longer the oral environment is acidic. Studies have clarified that it is not how much carbohydrate is present, but rather the acidity that is the main determinant for cavities risk.


By addressing the risk factors and controlling the chemistry, we are able to predictably prevent or stop demineralization in their early stages and help our patients avoid costly future dental interventions!
Reference:
- Pontefract H et al. The erosive effects of some mouth rinses on enamel. A study in situ. J Clin Periodontol. 2001 Apr;28(4):319-24.